|
Volume 15 Issue 2 Pages 68 -
127 (October 1998)
Citation: Zagrebelny, SV (1998) Morphological Characteristics Of Sea Otter Enhydra lutris L. (Carnivora, Mustelidae) Pelage And First Age Moult. IUCN Otter Spec. Group Bull. 15(2): 93 -102
Previous | Contents
| Next
Morphological Characteristics Of Sea Otter Enhydra Lutris L. (Carnivora, Mustelidae) Pelage And First Age Moult
Sergey Vladimirovitsh Zagrebelny1
1State National Preserve “Komandorsky”, Nikolskoe, Aleutian distr., Kamchatka, 684500, Russia
(received 4th September 1998, accepted 5th December1998)
|
Abstract: Skin samples were taken from the midback and stomach of sea otters from embryos, new-born, one-month pups and adult sea otters up to 8 years old, from the Commander islands. The pale yellow coat of the embryos was shorter and less dense on the stomach than on any other part of the body. Two types of hair, guard hairs and underfur,were determined on the otters’ back.
Generally, the fur of newborn and one-month old pups was still developing, and three different kinds of fur were noted - guard hairs, intermediate hairs and underfur. The cuticular scale pattern of juvenile guard hairs was of a mosaic type in the distal portion and lanceolate in the middle. The cuticle of the underfur and intermediate hairs was lanceolate shaped.
No significant differences in embryo and new born pup hair parameters were found. Therefore it is assumed that embryo fur pelage consists of two hair-types with the taller and denser fur of older pups belonging to the juvenile generation. Two types of adult hairs were identified, 3-4 guard hairs and two underfur hairs. The cuticle of the guard hairs was mosaic in style at the blade and lanceolate at the base. The cuticular scale of underfur hairs was lanceolate throuhout their length. The juvenile pelage changed at 2 to 5 months of age, beginning at the chest and groin. At six days the moult progressed from the surface of the stomach to the back, ending on the head and the rump. The overall moult covered 80-90 % of the animals’ skin and concluded by the fourteenth day. Juvenile hairs remained on the head and rump until six months of age. Secondary moulting was noted in the animal living in the house after 55-60 days of observation. The new generation of definitive pelage was thinner and longer, and the moult followed the same order as the first moult. We argue that the first adult moult following the juvenile moult is an adaptation to extreme temperatures. |
| Español |
INTRODUCTION
Previous researchers, including Barabash-Nikiforov (1938), Barabash-Nikiforov et al. (1968), Marakov and Sudakov (1978), have described the structure of the pelage in adult sea otter's hair. They described length topography, shape and some morphological features of both the guard and underfur hairs. Williams (1992) has also published some data on morphology, skin histology, hair density, length, diameter and structure and has examined variations in these features in different animals. In our previous investigation we attempted to describe juvenile and adult sea otter hair structure, inner and external features, and height topography of guard and underfur hairs. We also found variations with sex, age and season (Zagrebelny, 1993). In this article we further investigate the morphological characteristics of the pelage and first moult of both adult and young sea otters.
METHODS
Skin samples were taken from the midback and stomach of sea otters from embryos, new-born, one-month pups and adult sea otter (7-8 years old) from around the Commander islands (Kamchatka, Russia). Preparation and biometric calculations were made using standard techniques for microscopic and histological examination (Sokolov et al., 1988; Lakin, 1990).
Hair height was measured on dry skin using a graduated ruler. Hairs from embryos were examined after fixing fresh individual hairs to a glass surface and measured using a microscope micrometer. Order and timing of juvenile (first postnatal) and definitive (second postnatal) moult were obtained using two young sea otters. The first animal was captured at Medny Island in summer 1990, the second was kept at the authors’ home between February and April 1992. Observations were made on the first, sixth, fourteenth and thirtieth days after moult.
The cuticular structure was determined and measured using negative imprints in colourless varnish (Kuznetchov, 1952; Khmelevskaja, 1965).
RESULTS
Hair morphology of juvenile pelage
The light yellow pelage of the embryo was shorter and less dense on the stomach than on any other part of the animals’ body. For the most part, hairs were at the stage of growing the distal flat end. Two types of hair, guard hairs and underfur,were determined on the otters’ back.
Generally, the fur of newborn and one-month old pups was still developing. Three different kinds of fur were noted - guard hairs, intermediate hairs and underfur. The size of these hairs is shown in Table 1.
| Table 1:
Individual hairs characteristics of different sea otters at different ages |
| Age of Animal |
Hair Type |
No |
Hair length
(mm) |
Blade Width
(mm) |
Core
maintenance
% |
in blade |
| in shank |
|
| Back Fur |
| Embryo |
guard
underfur |
1°
6 |
4.1 ± 0.20
5.5 ± 0.34 |
62.35 ± 3.52
21.16 ± 1.91 |
24.90 ± 1.14
12.43 ± 0.46 |
| Newborn |
guard 1st
2nd
3rd
intermediate
underfur |
9
6
7
18
8 |
35.7 ± 0.44
20.5 ± 0.10
15.4 ± 0.65 |
69.60 ± 2.25
51.26 ± 3.74
55.04 ± 2.06
40.68 ± 1.28
41.36 ± 2.28
30.18 ± 1.90
27.26 ± 0.63
19.28 ± 0.89
17.67 ± 0.76
14.25 ± 0.49
|
31.78 ± 1.18
22.03 ± 1.30
34.87 ± 1.40
19.18 ± 0.67
27.76 ± 0.52
15.55 ± 0.38
25.58 ± 0.38
17.57 ± 0.29
21.47 ± 0.59
16.76 ± 0.29 |
| 1 month |
guard 1st
2nd
3rd
intermediate
underfur
|
6
10
14
10
6 |
42.3 ± 0.01
32.5 ± 0.72
31.3 ± 0.68
26.9 ± 0.59
25.3 ± 0.41 |
70.17 ± 3.11
46.30 ± 2.36
49.01 ± 2.06
33.76 ± 1.02
38.16 ± 2.06
28.57 ± 0.92
24.16 ± 1.10
20.13 ± 1.08
19.08 ± 0.83
14.57 ± 0.64 |
34.86 ± 1.01
18.78 ± 0.86
23.85 ± 0.68
17.18 ± 0.47
26.66 ± 0.84
15.04 ± 0.40
23.13 ± 0.53
19.92 ± 0.31
18.63 ± 0.08
8.82 ± 0.43 |
| 7-8 years |
guard 1st
2nd
3rd
underfur 1st
underfur 2nd
|
7
9
6
7
8 |
31.0 ± 0.26
30.1 ± 0.29
29.0 ± 0.41
28.7 ± 0.21
14.5 ± 1.20 |
129.6 ± 4.42
59.92 ± 4.86
82.33 ± 5.05
31.34 ± 1.72
41.17 ± 2.80
18.83 ± 0.92
13.66 ± 0.48
8.09 ± 0.16
10.75 ± 0.20
8.88 ± 0.03 |
19.72 ± 2.30
17.87 ± 4.03
14.37 ± 1.47
10.18 ± 0.92
7.93 ± 0.77
10.88 ± 0.98
16.42 ± 1.19
12.46 ± 4.65
18.90 ± 1.42
27.50 ± 1.80 |
| Stomach Fur |
| Embryo |
underfur |
15 |
7.2 ± 0.44 |
24.75 ± 0.17 |
23.57 ± 0.06 |
| Newborn |
guard 1st
2nd
intermediate
underfur |
5
13
12
8 |
29.9 ± 0.56
26.3 ± 0.70
22.0 ± 0.07
13.6 ± 0.41 |
23.12 ± 4.68
61.58 ± 6.75
39.37 ± 0.20
30.87 ± 0.23
25.04 ± 0.60
24.02 ± 1.25
18.58 ± 0.33
15.87 ± 0.76 |
29.77 ± 1.76
21.93 ± 1.73
30.94 ± 0.75
21.01 ± 0.59
24.36 ± 0.42
18.97 ± 0.29
22.44 ± 0.44
15.73 ± 0.09 |
| 1 month |
guard 1st
2nd
intermediate
underfur |
7
14
8
6 |
38.6 ± 1.68
29.9 ± 0.47
26.5 ± 0.39
21.1 ± 0.81 |
82.77 ± 2.97
60.93 ± 2.19
37.42 ± 1.46
30.37 ± 0.91
25.33 ± 1.18
25.58 ± 1.43
15.64 ± 0.95
15.11 ± 0.99 |
23.82 ± 4.18
11.78 ± 0.80
26.53 ± 0.78
17.84 ± 0.41
20.22 ± 0.56
15.63 ± 0.67
19.18 ± 0.46
16.58 ± 0.36 |
| 7-8 years |
guard 1st
2nd
3rd
4th
underfur 1st
underfur 2nd
|
7
7
7
7
7
8
|
28.4 ± 0.67
25.4 ± 0.39
24.6 ± 0.29
23.3 ± 0.18
23.2 ± 0.45
42.7 ± 0.88 |
133.70 ± 3.84
62.03 ± 3.13
89.14 ± 6.10
32.63 ± 3.07
51.31 ± 3.69
21.76 ± 1.67
26.19 ± 3.06
13.06 ± 1.08
13.00 ± 0.00
8.26 ± 0.29
10.94 ± 0.27
7.80 ± 0.00 |
9.93 ± 0.81
13.67 ± 1.51
8.68 ± 0.75
8.99 ± 0.82
5.95 ± 0.54
3.75 ± 1.45
9.38 ± 1.13
4.34 ± 1.90
13.72 ± 0.55
3.53 ± 1.75
12.14 ± 2.02
4.76 ± 3.18 |
|
The cuticular scale pattern of juvenile guard hairs was of a mosaic type in the distal portion and lanceolate in the middle (Fig.1). The cuticle of the underfur and intermediate hairs was lanceolate shaped. Their sizes are given in Table 2.
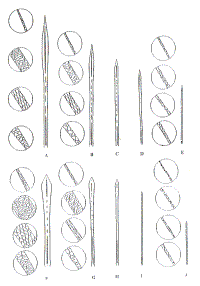 |
| Figure 1. Juvenile (A-E) and definitive (F-J) hair structure and cuticle structure in tip, blade, shank and root of hairs (click for larger version) |
| Table 2:
Cuticular cell size of juvenile and definitive hairs |
| Hair Type |
Characteristics |
shank |
blade |
| x |
Sx |
cv |
lim |
n |
x |
Sx |
cv |
lim |
n |
| juvenile |
| guard 1st |
length
width |
61.75
10.16 |
1.37
0.17 |
9.9
7.7 |
49.4 - 72.8
8.0 - 11.0 |
20 |
8.94
33.85 |
0.32
2.41 |
30.1 |
6.0 - 12.0 |
20 |
| guard 2nd |
length
width |
65.39
11.72 |
1.15
0.25 |
7.9
9.6 |
54.6 - 72.8
10.4 - 13.0 |
20 |
8.15
24.97 |
0.21
1.23 |
11.8
33.0 |
6.0 - 10.4
15.6 - 31.2 |
20 |
| underfur |
length
width |
62.77
7.90 |
1.52
0.02 |
10.8
1.3 |
49.4 - 75.4
7.8 - 8.0 |
20 |
44.33
7.93 |
0.83
0.08 |
8.4
4.3 |
39.0 - 52.0
7.8 - 8.0 |
20 |
| definitive |
| guard 1st |
length
width |
66.10
9.79 |
1.34
0.19 |
9.1
8.7 |
57.2 - 78.0
7.8 - 10.4 |
20 |
11.66
63.18 |
0.72
4.64 |
27.4
32.8 |
7.8 - 20.8
31.2 - 106.6 |
20 |
| guard 2nd |
length
width |
57.11
9.81 |
0.99
0.19 |
7.8
8.7 |
52.0 - 78.0
8.0 - 10.4 |
20 |
15.04
54.80 |
0.94
4.13 |
28.1
34.2 |
10.4 - 21.0
26.2 - 91.0 |
20 |
| guard 3rd |
length
width |
84.76
9.14 |
1.45
0.16 |
7.7
7.8 |
72.8 - 93.6
8.0 - 10.4 |
20 |
11.60
34.36 |
0.44
1.90 |
17.4
24.7 |
8.0 - 15.0
24.4 - 52.0 |
20 |
| underfur 1st |
length
width |
72.34
8.46 |
1.50
0.18 |
9.5
9.3 |
54.6 - 85.8
7.8 - 10.4 |
20 |
36.61
7.86 |
1.48
0.08 |
16.7
4.3 |
28.6 - 54.6
7.0 - 9.0 |
20 |
| underfur 2nd |
length
width |
89.16
8.41 |
1.35
0.16 |
6.8
8.6 |
80.6 - 104.0
7.8 - 10.0 |
20 |
43.16
7.82 |
1.62
0.07 |
16.8
4.2 |
33.8 - 52.0
7.0 - 7.8 |
20 |
|
No significant differences (P>0.05) in embryo and new born pup hair parameters (blade width, maintenance of core in the blade or length of cuticular cells) were found. Therefore it is assumed that embryo fur pelage consists of two hair-types with the taller and denser fur of older pups belonging to the juvenile generation.
Changes in the height of the pelage over different parts of the otters’ skin surface was used to indicate the process of juvenile hair growth (Table 3). The condition of the embryo pelage was better on the back, with stomach pelage being less dense and consisting of underfur only. The fur of new-born cubs was denser and higher and continued to grow, as indicated by the increase in length of the guard and underfur hairs of one month old pups. The fur of new-born and one month old pups seems to be responsible for thermoregulation although, in new born pups, there was only underfur hairs on the chest.
| Table 3:
Height of guard (1) and underfur (2) hairs at different locations |
| Age of animals |
Number of animals |
head |
withers |
back |
rump |
throat |
chest |
stomach |
| 1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
| embryo |
1 |
10.2 |
11.7 |
8.0 |
11.0 |
7.7 |
9.5 |
9.0 |
9.8 |
- |
4.6 |
- |
3.3 |
- |
7.2 |
newborn |
2 |
28.0 |
17.0 |
32.0 |
17.5 |
30.5 |
16.0 |
28.5 |
19.0 |
13.5 |
8.5 |
- |
12.0 |
23.5 |
14.4 |
one-month |
2 |
30.0 |
18.0 |
41.0 |
21.0 |
39.5 |
20.5 |
41.5 |
23.0 |
22.0 |
16.0 |
24.0 |
15.5 |
40.0 |
23.0 |
2-8 vear |
6 |
25.0 |
13.8 |
34.5 |
21.7 |
28.7 |
21.8 |
28.3 |
20.0 |
24.7 |
14.0 |
25.5 |
11.3 |
27.3 |
18.5 |
|
Hair morphology of definitive pelage
Two types of adult hairs were identified, 3-4 guard hairs and two underfur hairs (Zagrebelny, 1993) although Marakov and Sudakov (1978) noted six kinds of guard hairs, intermediate and two kinds of underfur. The underfur hairs were distinctly different in both length and core maintenance, whereas the second kind of underfur hairs had no core except at the blade (Table 1).
The cuticle of the guard hairs was mosaic in style at the blade and lanceolate in the shank. The cuticular scale of underfur hairs was lanceolate throuhout their length (Figure 1). The length of the cuticular petal increased from guard to underfur hairs whilst the width decreased (Table 2).
First postnatal moulting
The juvenile pelage changed at 2 to 5 months of age (Barabash-Nikiforov, 1968; Marakov and Sokolov, 1978; Kenyon, 1969). The pelages of both animals studied was similar: moulting was noted at the head, withers (back of the neck), on the forearm in particularly, the back, stomach, tail, side and foot. Moulting was complete on the chest, inner surface of the forearm and the groin (Figure 2).
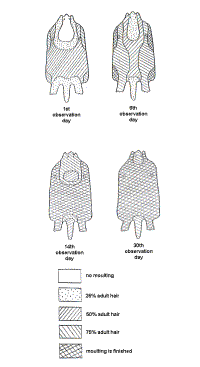 |
| Figure 2 . Moult order of juvenile hairs. (click for larger version) |
Visual observations indicated the beginning of the moult on the chest and groin. At six days the moult progressed from the surface of the stomach to the back (Figure 2), ending on the head and the rump. The overall moult covered 80-90 % of the animals’ skin and concluded by the fourteenth day. Juvenile hairs remained on the head and rump until six months of age.
Secondary moulting was noted in the animal living in the house after 55-60 days of observation. The new generation of definitive pelage was thinner and taller, whilst the colour of the fur became lighter. The order of moult was the same as in the first postnatal (juvenile) moult: back and stomach first and, within 17-20 days, the moult spreading from the back to the withers, forearm, side and from the stomach to the groin and hips. The head, shoulder blades and rump moulted last.The animal was released to the wild before moulting process was finished. The animal died shortly after it was released.
Discussion
The sea otter is the smallest marine mammal. It lives in cold water and, though it has no insulating fat layer, it does have very dense fur. This fur has an underlayer which traps air and thus enables the otter to maintain its’ body heat (Sokolov, 1973; MacArthur, 1989; Williams, 1992). Room temperature (20-25 °C) is critical (a temperature beyond which death occurs due to overheating) for animals (Morrison et al., 1974). Sea otters can lose body heat to the outside environment in two ways. Firstly, wetting of the fur can result in an increase in heat production of 3 - 5.5 times (Costa and Kooyman, 1982). Secondly, through the changes in pelage structure (i.e. moulting) as observed during this study. We argue that the first adult moult following the juvenile moult is an adaptation to extreme temperatures. This may also be the process for the seasonal moult.
This study identified some peculiarities of pre- and post-natal fur development. Embrionic fur was sparse and short and we separated it into two types of hairs; guard and underfur. The stomach of the embryo was covered with underfur hairs only. New-born and one-month old pup pelage was dense and high and protected the pup's bodies from the cool ocean water by a layer of air beneath the fur. All types of hair identified in the adult pelage were also found in the pelage of young otters, i.e. two kinds of guard hair, intermediate hairs and underfur.
This study indicated the order of juvenile (first postnatal) and adult (second postnatal) moulting. In all instances the moult began on the stomach, followed by the back, withers and rump. The moult started on that part of the body with the shortest hairs (or with latest hair development), followed by parts with the longest pelage. This process was similar to that found by Zagrebelny (1993) using hair height topography pictures. Further, the same rule was noted by Ivanter et al. (1984) for other semi-aquatic mammals, e.g. Neomys fodiens.
REFERENCES
Barabash-Nikiforov, I.I. (1938). The sea otter and stages in its investigation. Nature 2: 51-61 [In Russian]
Barabash-Nikiforov, I.I., Marakov, S.V., Nikolayev, A.M. (1968). Kalan (sea otter). Moscow. Vycshaya Shkola. 154 pp [In Russian]
Costa, D.P. & Kooyman, G.L. (1982). Oxygen consumption, thermo-regulation and the effect of fur oiling and washing on the sea otter Enhydra lutris. Can. J. Zool. 60: 2761-2767.
Ivanter, E.V., Ivanter, T.V. & Levina, R.V. (1984). The adaptive peculiarities of the structure of hair cover and of the moult in semi-aquatic mammals, Neomys fodiens taken as an example. J. Zool. 63: 245-255. [In Russian]
Kenyon, K.W. (1969). The sea otter in the Eastern Pacific Ocean. North. Amer. Fauna 68: 352.
Khmelevskaya, N.V. (1965). Structure of the rodent hair cuticle, its variability and its significance for taxonomy. J. Zool. 44: 1064-1073.
[n Russian]
Kuznetchov, B.A. (1952). Trade testing principles of fur row materials. Moscow. Zagotizdat. 321 pp. [In Russian]
Lakin, G.F. (1990). Biometry. Moscow. Vycshaya shkola., 335 pp. [In Russian]
MacArthur, R.A. (1989). Aquatic mammals in the cold. Advances in Comparative and Environmental Physiology. Berlin ets. 4, pp. 289-325.
Marakov, S.V. & Sudakov, V.V. (1978). On some morphological adaptations of the sea otter. pp.117-119. In: Marine Mammals. Thesis of the 7th All-Union meeting. Moscow. [In Russian].
Morrison, P., Rosenman, M. & Estes, J. (1974). Metabolism and thermo-regulation in the sea otter. Physiol. Zool. 47: 218-229.
Sokolov, V.E. (1973). Skin pelage of mammals. Moscow, Nauka, 487 pp. [In Russian]
Sokolov, V.E., Skurat, L.M., Sumina, E.B. & Schabadash, S.A. (1988). Guide to the study of mammal skin pelage. Moscow, Nauka, 280 pp. [In Russian]
Williams, T.D. (1992). An analysis of Californian sea otter (Enhydra lutris) pelage and integument. Marine Mammal Science 8: 1-18.
Zagrebelny S.V. (1993). Pelage of the sea otter Enhydra lutris (Carnivora, Mustelidae): hair structure, topography and some adaptive features. J. Zool. 72: 129-140 [In Russian]
Resúmen:Características morfológicas del pelaje y primera muda de la nutria marina Enhydra lutris L. (Carnivora, Mustelidae).
Se tomaron muestras de la zona dorsal media y el estómago de embriones de nutrias marinas, neonatos, crías de un mes y adultos de 7 a 8 años de las islas Commander. El pelaje amarillo pálido de los embriones fue más corto y menos denso en el estómago que el cualquier otra parte del cuerpo. En la mayor parte de este el pelo se encontraba en crecimiento. En la espalda se determinaron dos tipos de pelo: guardianes y felpa. Generalmente el pelaje de neonatos y crías de un mes continuaba en crecimiento, y era posible distinguir tres tipos de pelo: guardianes, intermedios y felpa. Las escamas cuticulares de los pelos guardianes de juveniles presentaban un patrón de tipo mosaico en la zona distal, y lanceolado en la región media. La de los pelos intermedios y felpa, tenía un patrón lanceolado. No se detectaron diferencias en las características de los pelos de embriones y neonatos, por lo que se asume que el pelaje de los embriones consiste en dos tipos de pelo, y que el pelaje mas largo y denso de crías mayores pertenece a la generación juvenil. En adultos se distinguieron dos tipos de pelos: tres a cuatro guardianes y dos de felpa. La cutícula de los pelos guardianes de adultos presentaba un patrón tipo mosaico en la parte de la hoja y lanceolado en la base. El de la felpa era lanceolado en todo el pelo. El pelaje juvenil cambió a los 2 a 5 meses de edad, comenzando por el pecho y la ingle. A los 6 días el proceso cubría desde la superficie del estómago hasta la espalda, terminando en la cabeza y las ancas. La muda general cubría el 80 al 90% de la piel y estaba concluida hacia el 14º día. El pelaje juvenil se mantuvo en la cabeza y las ancas hasta los 6 meses de edad. La muda secundaria fue observada en animales domesticados tras 55 a 60 días de observación. El pelaje definitivo es mas fino, largo y claro, y el proceso de muda sigue el mismo orden que el de la primera. Consideramos que la primera muda adulta que sigue a la juvenil es una adaptación a las temperaturas extremas.
Vuelva a la tapa
Previous | Contents | Next
|


